The makers of the first NASH drug that has met the goals set by FDA, Ocaliva by Intercept, have once again been told that if they spend another billion dollars they can apply again. Intercept is beaten and will withdraw.
The FDA has become dysfunctional in its handling of drug applications regarding liver disease. I gave patient testimony at a recent FDA public meeting discussing this drug.
Let me set the stage. I know personally, some of the top NAFLD/NASH doctors in the world who recommended approval of this drug. The patients are universally in favor. The FDA voting panel consisted of people who had little real knowledge of the disease but were there to put the FDA staff opinions into the public record. ( That isn't what they claim, of course, just my opinion ). That seems clear, as there was no one on the panel who was an expert on liver disease and the panel voted against the drug which now echos in the official FDA decision.
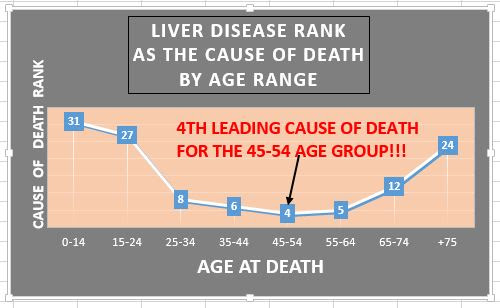
So what is the state of the patient community? Liver disease is the 4th leading killer of people in their 40's and 50's. Little known statistic but scary. It isn't the old drunk guy problem any more.
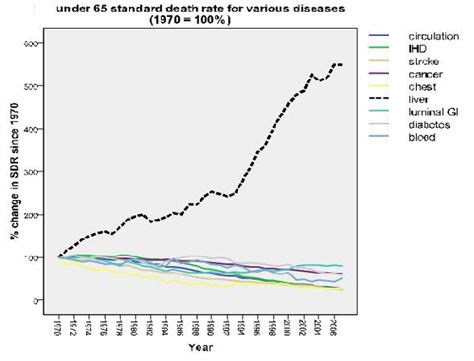
If we look at how medicine is doing against most diseases we can chart the rate of change in death rate over time. For most conditions we are making slow but steady progress, but that black line is liver death. We are losing that war badly. Remember that is the rate of change, people are dying steadily faster with liver disease.
Overseeing this mess are our guardians at the FDA. One might think this would be a vital concern, but one would be wrong. The argument put forth by the FDA staff at the public meeting was that it was possible that there could be one death per 1,000 patient years from the use of this drug Ocaliva. OK a data point to consider. The drug trial showed that perhaps a quarter of patients would benefit from taking it and reduce their fibrosis by one stage. Generally we think that one stage means 7 years so even if they relapse it is a win and not everyone would relapse.
As a point of comparison, the FDA approves high-priced cancer drugs that don’t even extend life for 10 weeks. For instance, the 72 cancer therapies approved between 2002 and 2014 only bought patients an extra 2.1 months of life compared with older drugs.
A cynic might wonder how such a strange thing could happen. When we step back a little you can see that the tree of life has a lawyer hanging from every limb and there is a disturbing crowd of lawyers hanging around the bottom looking for a place to grab on and suck a bit of life out of the system. Can this behavior be caused by the counsel or fear of lawyers by FDA? We know that no decisions are made without the guidance of a lawyer.
A cynic might say that, but I won't, I won't.
We know that Madrigal has a better drug coming before FDA soon but that still puts us more than a year away from any possible therapy. Thousands more will die while we wait. I can't help but wonder whose lawyers prefer simpler times or might FDA's lawyers prefer certain death over the publicity of one extra death per thousand patient years inviting hunting lawyer scrutiny.. We will never know as rule number one is don't talk about things with patients.
For me, the most important thing about the intercept drug was that by showing that for some people it could reduce fibrosis it proved that for a larger group it would be able to slow or stop progression. For someone who has not yet become a cirrhosis patient that is the most important thing. I shouldn't have to get really sick before you pay attention to my problems.
Now that we yet again won't see a therapy it is even more important that we speak up. I see that thousands of you still have not yet taken the survey, The State of NAFLD/NASH Care in America. Do you think organizations like FDA will pay attention if there is little evidence that you care?
Do your part and take 20 minutes to help.